
THE SCIENCE BEHIND BAKING SODA
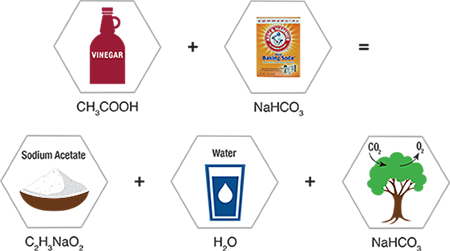
Baking Soda is a White Crystalline Powder also Known as Sodium Bicarbonate, which Acts as a Buffer to Neutralize Odor.
- When added to acid solutions, a buffer raises their pH.
- When added to alkaline solutions, it lowers their pH.
- It always tries to bring whatever solution it is in to its own pH.
- Baking soda has a pH of 8 which is slightly alkaline.
Most Odors Come from Either Strong Acids Like Sour Milk or Strong Bases Like Spoiled Fish.
- Food odors resulting from either acidic or basic compounds can be neutralized with baking soda into more neutral and odor-free salts.
- Baking Soda can also deodorize when it’s dissolved in water.


